Electrostatic Force – Coulomb’s Law Notes
- Electrostatic force is between charged particles that aren’t moving
- The forces are always equal & opposite forces (Newton’s 3rd Law)
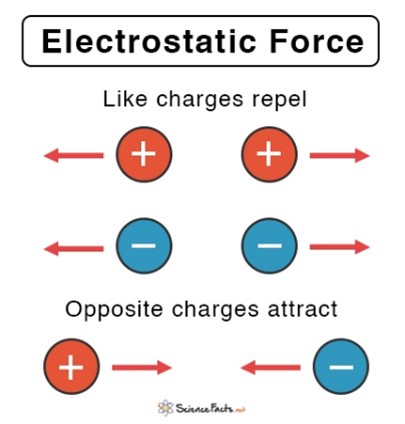
- If the charges are different (one positive and one negative), the forces are attractive
- If charges are the same, the forces are repulsive
Coulomb’s Law
![]()
Fc = electrostatic force (N)
k = Coulomb constant = 9.0 x 109 Nm2/C2
q1 and q2 = charges (C)
r = distance between the particles (m)
Force is proportional to either one of the two charges.
(If you double a charge, the force becomes twice as strong)
Force is proportional to the inverse square of the distance between the particles.
(If you double the distance, the force becomes ¼ times as much as before, or 4 times weaker)
This law is very similar to the Universal Law of Gravitation, except we have two charges instead of two masses.
The constant in Coulomb’s Law is much, much larger than the Gravitational Constant. This tells us that the electrostatic force is much stronger than gravity.
Charge Signs, Absolute Value, and Force Direction
- The equation is typically written with absolute value around q1 and q2: F = k|q1q2| / r2
- This means you can ignore the charges in the math. Make your force a positive result – this numerical result is the magnitude of your force vector.
- The direction of the force depends on where the particles are located in relation to each other whether the charges attract (different charges) or repel (like charges).
- To put all of this another way: don’t try to use the product of your +/− signs to determine the direction of your force. Instead, use your knowledge of attracting or repelling charges to figure that out.
Units of Charge
- Electric charge units is the Coulomb (symbol is C)
- A Coulomb is a large amount of charge, so you will often see smaller units instead.
- nanoCoulomb: 1 nC, is = 10-9 C
- 1 microCoulomb: 1 µC = 10-6 C
- Convert units to Coulombs before you do any math.
- Example: if the charge is 7.0 nC, you would instead substitute (7.0 x 10-9 C) into your equation.
- Tip: Use the “E” or “10^” button on your calculator to avoid errors with scientific notation.
Other Coulomb’s Law units tip: the distance must be in meters. Convert to meters before doing the math.