Binary Ionic Compounds are substances formed from two different elements bonded together.
Compounds are neutral. They have an overall charge of zero.
These compounds have one positive ion (usually a metal) and one negative ion (usually a non-metal). The metal is written first in the formula.
Examples of Ion Compound Formulas:
CaO
Na2S
Al2O3
The subscript numbers tell us how many of each ion are present in the compound. These tell us the ratio of one element to the other.
If there is no subscript, that means the subscript is one.
Ionic compounds are also called salts in chemistry. Table salt, NaCl, is just one type of salt.
Example: Lithium and Fluorine
- lithium forms Li+ ions (+1 charge: the “1” is not written)
- chlorine forms F− ions (-1 charge: the “1” is not written)
- Formula of the compound is LiF
- They form a 1:1 ratio, because the +1 and -1 charges balance out to a net charge of zero.
(See the notes for figuring out those charges)
To see why this combination works out well, look at electron box diagrams for each element.
| Li (neutral atom) | F (neutral atom) |
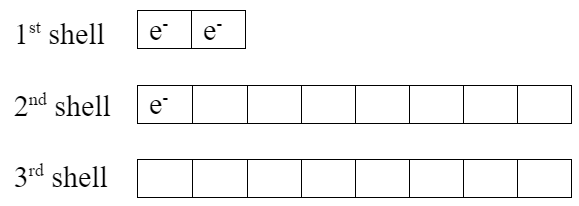 |
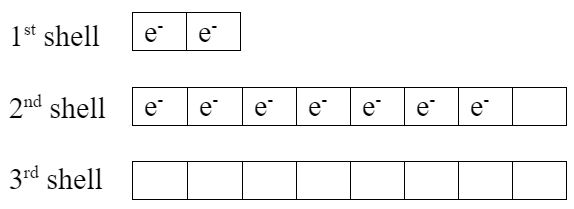 |
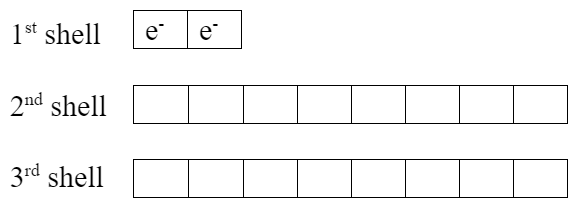
Lithium has one extra valence electron. Fluorine is missing one valence electron. Both elements would be more stable if lithium gives its valence electron to fluorine, which makes them both become ions:
| Li+ ion | F− ion, fluoride |
 |
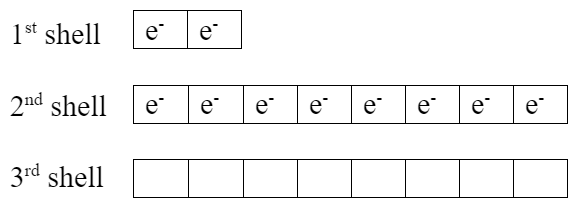 |
- Notice the exactly full valence shells.
- Lithium ion now has the same electron configuration as helium.
- Fluorine ion now has the same electron configuration as neon.
- Both elements now have noble gas electron configurations, which are very stable with their full valence shells.
Thus, ionic compounds tend to be very stable, and their ionic bonds are very strong. This means it takes a lot of energy to break them, which causes ionic compounds to have high melting and boiling points.