Light is Electromagnetic Radiation.
Light travels at the speed of light, which is called c.
c = 299,792,458 m/s
(This number is perfectly exact by definition, because the meter unit has been redefined so that this speed is exactly correct)
Or, approximately:
c ≈ 3.00 x 108 m/s
You can calculate the wavelength of light given its frequency or vice versa:
c = λf
Where:
c = speed of light (m/s)
λ = wavelength (m)
f = frequency (Hz, or s−1)
Light has wave properties and behaviors and also particle properties and behaviors.
Wave Properties of Light
- Different wavelengths and frequencies of light can be observed.
- Different wavelengths of light are different colors.
Electromagnetic Spectrum
Light waves of different wavelengths / frequencies / energies give us the different types of light.
We can only see a small part of the spectrum, which includes wavelengths of about 700 nm (red) to 400 (violet). The other parts of the spectrum, such as microwaves and gamma rays, are the same type of “stuff.” They are just different wavelengths of light that our eyes are not adapted to see.
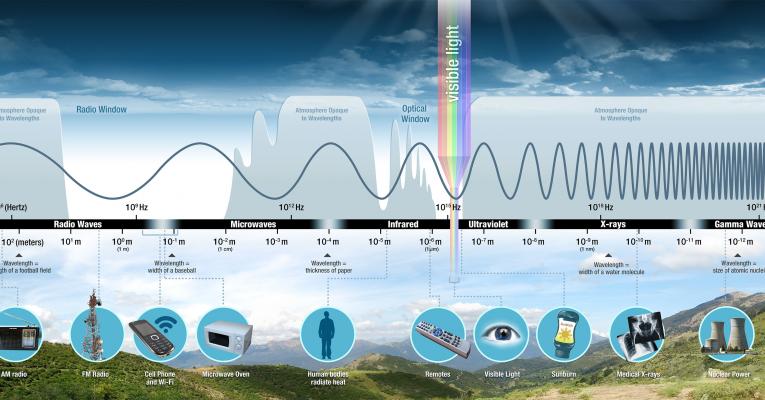
Image is from NASA’s page on EM Spectrum
Wave Behaviors of Light
- Light waves reflect.
- Light waves refract (change direction/angle) when they enter a different material with a different effective speed of light. Light Refraction makes it possible to make technologies like lenses and fiber optic cables, and more broadly it leads to the area of Optics.
- Light waves can interfere with each other: they pass through each other instead of colliding and bouncing backwards, and they also produce interference patterns in experiments when they interfere constructively and destructively.
- Light diffracts, or spreads out in a circular wave pattern when it is passes through a narrow opening in a barrier.
- Light has a Doppler Effect; if we move toward a light source, the apparent frequency increases and the light appears “more blue.” If we move away from a source, the light has a lower apparent frequency and appears “more red.” This has big implications for cosmology, because we observe that everything far away from the earth is moving away from us (everything is red shifted). This implies that the universe is expanding.
- Light’s wave behavior can be shown using a double slit diffraction experiment. Laser light from two narrow slits diffracts and interferes to create an interference pattern, which shows up as alternating bands of light and dark on a detector screen.
- You can learn more about double slit diffraction (Young’s Experiment) here, here, here, here, here, and here.
- One more link on light diffraction and interference – this one is from OpenStax High School Physics online textbook.
Particle Behavior of Light
- The smallest amount of light is a photon.
- A photon is a particle of light. It is a sort of wave packet.
These statements about light are a first step toward the idea of quantum mechanics. “Quantum” is a word that describes things that cannot be infinitely divided – there is a minimum fundamental size or amount for things that are “quantized.”
In most cases, a wave model of light adequately explains the observed experimental results that we see when we study light. However, there is one key area where the wave model doesn’t work out – Photoelectric Effect.
Photoelectric Effect
The Photoelectric Effect is what happens when light knocks electrons out of atoms of a metal. The emitted electrons are called photoelectrons.
You can look at a PhET simulation to get a quick idea of it.
Here’s a 2 minute video explanation of this concept.
When scientists carry out these experiments, they can change the frequency (color) of light shining on the metal and the intensity of the light. Then they observe how many photoelectrons are emitted (measure the current in the output wire) and how much energy those electrons have on average (measure the voltage in the output wire).
If light is all waves, then we would predict that increasing the frequency of light would increase the number of photoelectrons emitted, and we would expect that increasing the amplitude (intensity) of the light would increase the energy of the electrons. In fact, the opposite happens – experimentally, we can observe that increasing the frequency causes electrons to have more energy, and increasing the intensity causes more electrons to be emitted.
In other words, the experimental results for Photoelectric Effect experiments do not support a wave model of light. The wave model gets it backwards.
The results can make sense if we think of light as individual photons (particle model). One individual photon hits one individual electron, and it gives that electron more energy if it is a higher frequency / higher energy photon. Also, increasing the intensity of the light just means there are more photons of the same energy, so it makes sense that we would see more ejected electrons with the same average energy when we shine brighter light of the same color.
Key Takeaway: Photoelectric Effect experiments provide strong evidence that light is made of photon particles.
Interesting fact: Explaining the Photoelectric Effect earned Einstein his Nobel Prize. It was an important step toward the new study of quantum theory, or the study of the unusual behavior of the universe at very small scales.
Photon Energy
The energy of an individual photon can be calculated as follows:
![]()
Where:
E = energy of a photon (Joules)
h = Planck’s Constant = 6.626 x10−34 (J⋅s)
f = frequency (Hz, or s-1)
λ = wavelength (m)
c ≈ 3.00 x 108 m/s
This equation came about when Max Planck was trying different assumptions to solve the so-called Ultraviolet Catastrophe. In short, the wave model based equation for predicting black body radiation was giving absurd results, but adding an assumption that light is quantized, or made of individual particles, fixed the problem.
Photon Momentum
A photon of light has no mass.
Interestingly, a photon actually does have momentum.
![]()
Where:
p = momentum of photon (kgm/s)
h = 6.626 x10−34 Js
λ = wavelength of light (m)
This is a fascinating equation, because it ties together wave properties (wavelength) to particle properties (momentum) in light.
Light having momentum means that it’s possible to accelerate an object by shining light on it. Light’s momentum is very, very small, so it takes a lot of light, but it’s still possible. In fact, some concepts for future space exploration involve creating extremely light spacecraft with very high surface area and then using earth lasers or orbital lasers to accelerate them to very high speeds.
Bonus: Particle – Wave Duality of Other Particles
To take the previous ideas a step further, other particles besides photons have particle-wave dual nature. This includes electrons and protons.
These types of particles also show wave behaviors such as interference. Scientists do double slit diffraction / interference experiments with electrons, similar to the experiments with light, and they are very important and interesting.
Here’s one nice video from Arvin Ash about these types of experiments. If you like that one, here’s another one that’s perhaps even crazier.
The same equation from above can be used to calculate the wavelength of a particle of a particle such as an electron (called the DeBroglie wavelength):
![]()
Where:
p = momentum of photon (kgm/s)
h = 6.626 x10−34 Js
λ = wavelength of light (m)