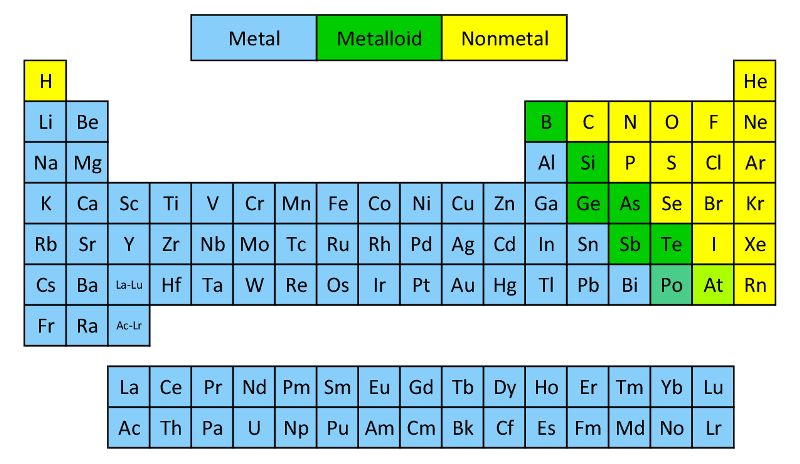
As you can see from this color coded version of the Periodic Table, most of the elements are metals. Only about 17 elements are nonmetals.
Metalloids are the six or so elements with properties of both metals and nonmetals. They lie along a staircase between the metals and nonmetals.
Some elements are hard to classify as metal, nonmetal, or metalloid; for example, some chemists consider astatine (At) a nonmetal while others call it a metalloid.
Metallic Character comes from an element’s tendency to lose electrons and form positive ions (cations). Elements that lose electrons easily are most metallic. These metallic elements have weaker Coulombic attractions between nucleus and outer electrons. Elements with strong Coulombic forces holding their outer electrons close are less metallic, and they tend not to lose their outer electrons. Nonmetals are in fact more likely to gain electrons from other atoms instead.
Properties of Metals
•Shiny (metallic luster)
•Conducts heat and electricty well
•Ductile (can be stretched out)
•Malleable (can be compressed/flattened)
•Reactive with acids
•High density
•Forms positive ions (cations)
•High melting points
Many metals are exceptions to these trends. For example:
•Gold doesn’t react with acids
•Lithium has a low density
•Lead has a dull appearance
•Cesium has a low melting point
Properties of Nonmetals (opposite of metal properties)
•Dull appearance (dull luster)
•Conducts poorly
•Brittle (opposite of ductile/malleable; breaks instead of bending)
•Low density
•Can form both anions (negative charge) and cations (positive charge)
•Low melting point
Nonmetals have exceptions too; for example:
•Carbon has the highest melting point of any element
•Carbon conducts electricity (but not as well as metals)
•Selenium is denser than many metals
Elements that have both strong metallic and nonmetallic characteristics are metalloids.